Corporate


With drug counterfeiting on the rise and regulatory deadlines fast approaching, the pharmaceutical supply chain will soon be at a critical point as it strives to ensure patient safety. From drug manufacturers to distributors, all supply chain partners will need to make crucial decisions to upgrade their production lines to comply with the Drug Supply Chain Security Act (DSCSA), Falsified Medicines Directive (FMD) and emerging market traceability deadlines.
Working closely with its technological industry partners to help support its customers in advance of meeting both U.S. DSCSA and EU FMD implementation dates, outsourcing provider PCI recently announced a significant expansion of its serialization capability, tripling capacity across its global supply network.
PCI has been actively serialising commercial products for both domestic and international markets for the past five years, with products earmarked for North America and Europe, in addition to emerging countries and markets such as South Korea, China and Brazil. As a whole, PCI works with more than 250 customers in packaging suites at 17 sites in the USA and the UK, supporting more than 8 thousand packaged medicine products destined to more than 100 global countries.
PCI has been using Marchesini equipment for many years, including labellers and cartoners.
BL A420 Track & Trace and labelling machine has been created by Neri Division to fully exploit its outstanding ergonomics: thanks to the work area that is separate from the mechanical and electrical parts, management and maintenance operations are very simple. All these top features make the BL A420 a truly high-tech yet very user-friendly machine.
The cartons are fed and accumulated in-line on the inlet belt to ensure a continuous flow of products, ensuring perfect code printing and tracking, even at high speed. A patented timing device positions and sets the cartons apart correctly on the toothed belts with adjustable opening, which also prevent slipping. The function of the timing device is to set the cartons at the correct distance apart; its special feature is that it is synchronised with the speed of the whole conveyance system and there is no need for any size change-over adjustments.
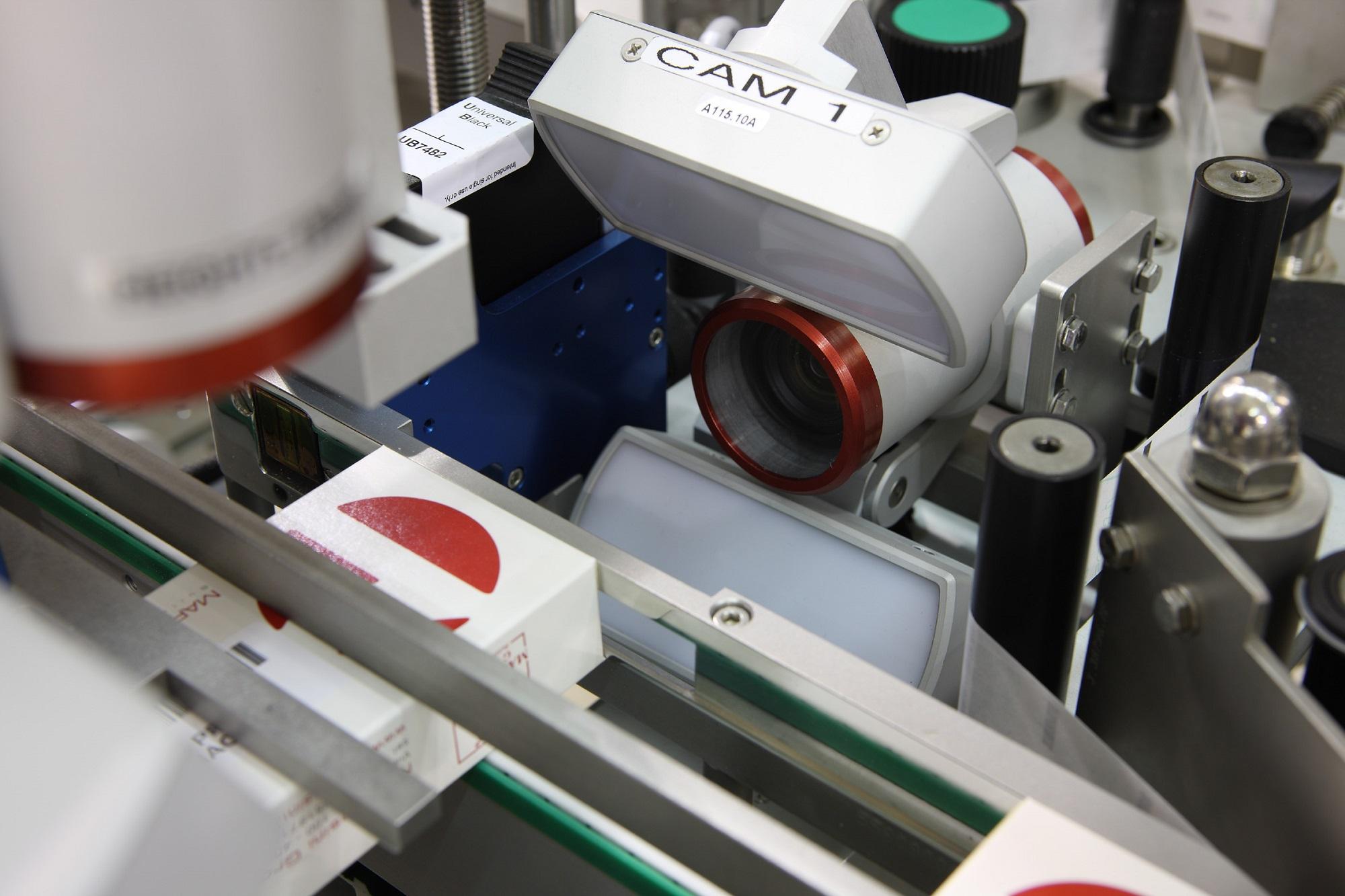
The first operation to be performed after the cartons have been positioned, is that of applying the vignette on the upper panel of the cartons; then, the unique 2D code (Datamatrix) and the corresponding human readable data are printed on the side flaps and sometimes on the upper panel. The unique code identifies and makes it possible to track each carton.
A camera then verifies both the codes and the readable data. Once the code has been completed in this way, two self-adhesive Tamper Evident seals are then applied on the corner closing points of the cartons.
All the label dispensing units are driven by servomotors, whose speeds are synchronised automatically with those of the carton conveyance system.
During these phases, the use of the “Fail Safe” operating logic means that only “correct” cartons are sent to the machine downstream, while the others are identified and rejected.
The Track & Trace equipment installed on the BL A420 consists of an inkjet printing system to print the 2D code and variable data on the lateral flap or on top side of the carton; the vision system developed by the Italian company Sea Vision consists of two cameras that check the 2D code print grading and the correct application of the vignette.
The BL A420 is incredibly flexible and can thus accommodate all the printing and vision systems available on the marketplace, plus it can be personalised according to the various regulations in force around the world.
This peculiarity makes the BL A420 the most suitable and complete machine for labelling and for serializing cartons, to assure that all the pharmaceutical products cartons have a unique identity.
The output of the BL A420 is up to 400 carton per minute.