Corporate






With drug counterfeiting on the rise and regulatory deadlines fast approaching, the pharmaceutical supply chain will soon be at a critical point as it strives to ensure patient safety. From drug manufacturers to distributors, all supply chain partners will need to make crucial decisions to upgrade their production lines to comply with the Drug Supply Chain Security Act (DSCSA), Falsified Medicines Directive (FMD) and emerging market traceability deadlines.
Working closely with its technological industry partners to help support its customers in advance of meeting both U.S. DSCSA and EU FMD implementation dates, outsourcing provider PCI recently announced a significant expansion of its serialization capability, tripling capacity across its global supply network.
PCI has been actively serialising commercial products for both domestic and international markets for the past five years, with products destined for North America and Europe, in addition to emerging countries and markets as South Korea, China and Brazil. In total, PCI works with more than 250 customers in packaging suites at 17 sites in the USA and the UK, supporting more than 8 thousand packaged medicine products destined to more than 100 global countries.
QUESTION: What is your mission?
ANSWER: PCI has five commitments to our customers:
- provide the industry’s leading customer experience;
- provide the industry’s highest quality and most flexible and reliable service;
- provide the best solutions to solve our customer’s difficult drug development problems;
- create a culture of engagement, ownership, and shared commitment to excellence in all we do;
- operate our business in a disciplined, responsible, and ethical fashion while delivering superior returns over time.
Q. What kind of services do you offer?
A. PCI Pharma Services is an integrated full service provider, taking compounds from the earliest stages of development through to a successful commercialization. We offer drug development, clinical trial services, commercial drug manufacturing and packaging and serialization services to Pharma and biotech companies.
Q. What do you do in terms of Corporate Social Responsibility?
A. Through our Corporate Social Responsibility commitment, we encourage associates to participate in activities and local initiatives and they generously donate our time, talents, and resources to local initiatives within the communities that we live and work. In fiscal year 2017, PCI has donated over $75,000 to local charities and community revival efforts. Including the Rockford Area Economic Development Council, local animal shelters, food pantries and Boys and Girls Club.
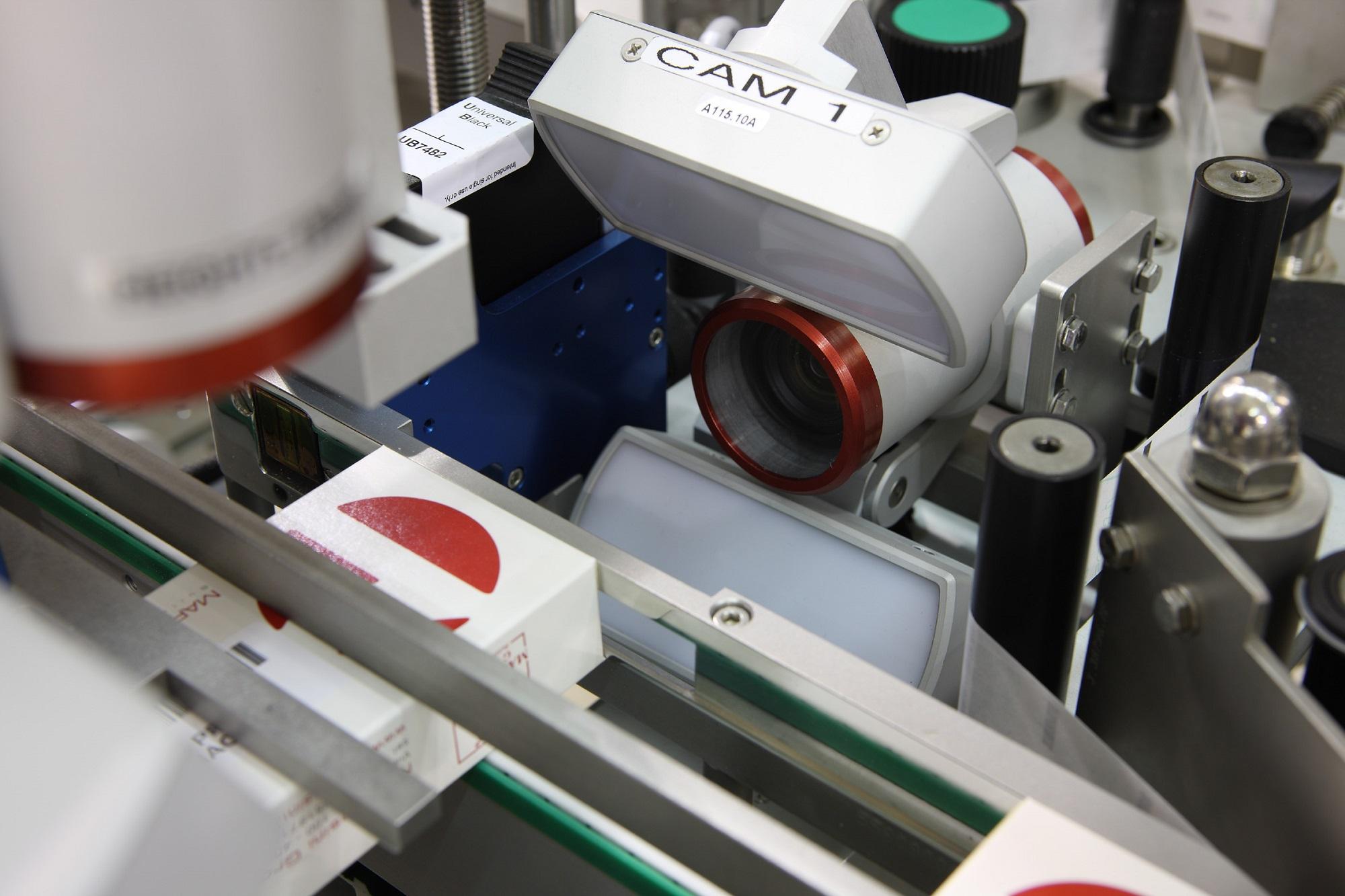
Q. Let’s talk about serialization implementation. What has been done and what are your next challenges?
A. We have been actively serializing commercial products for both domestic and international markets for the past five years, with products destined for North America and Europe, in addition to emerging market geographies such as South Korea, Turkey, Brazil, China and others. The world has woken up to the fact that serialization is happening and because of that, demand for equipment is very high and there has been a general increase in overall lead times.
We had been working with Antares and ended up going for a multi-source approach where we could find another serialization equipment provider and continue to use the interface of Antares. This would allow us to have a secondary machine supplier, all integrated with Antares software controlling the print and vision. We wanted to create a scenario where we could have similar equipment across all locations where you could potentially move the serialization equipment to the different packaging lines, which we call a “mobisuite”. This would allow a print and check, top view system, and a manual station to be moved to packaging lines.
We ended up having an order for 15 machines with six different specifications for machines. What Marchesini was able to do was work with us to collapse the specification requirements to two different designs and essentially created a factory within their factory with a team of engineers dedicated just to PCI. This was able to provide a lead-time two months ahead of the originally quoted lead-time. We now have orders for 31 machines within these specs.
